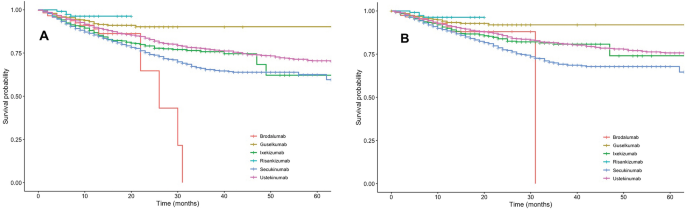
23/3/ IL7expanded Tγδ17 were treated with two different JAK2 inhibitors AZD1480 and TG At concentrations that inhibited IL23mediated pSTAT3Y705 (Fig 6A, left), both JAK2 inhibitors abolished IL23induced pRLCS (Fig 6A, right) Boehringer says antiIL23 drug beats Stelara in psoriasis trial Interleukin23 inhibitor outperforms Johnson & Johnson's blockbuster New data from a phase II trial of Boehringer Ingelheim's psoriasis candidate BI back up earlier results showing it is more effective than a rival drug from Johnson & Johnson After nine months' treatment30/3/21 At 18 months, IL23 inhibitors were the only drugs with a cumulative probability of drug survival above 90% 964% for risankizumab, and 911% for guselkumab;

Lilly S Il 23 Drug Beats Novartis Cosentyx In Plaque Psoriasis Fiercebiotech
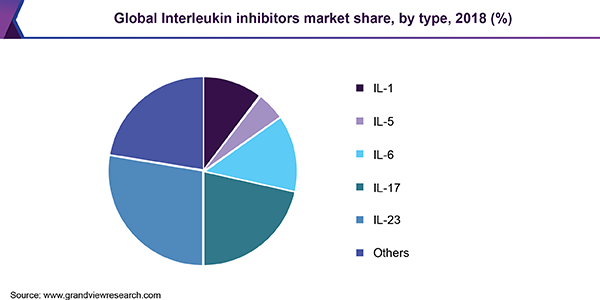
Il 23 inhibitor drugs
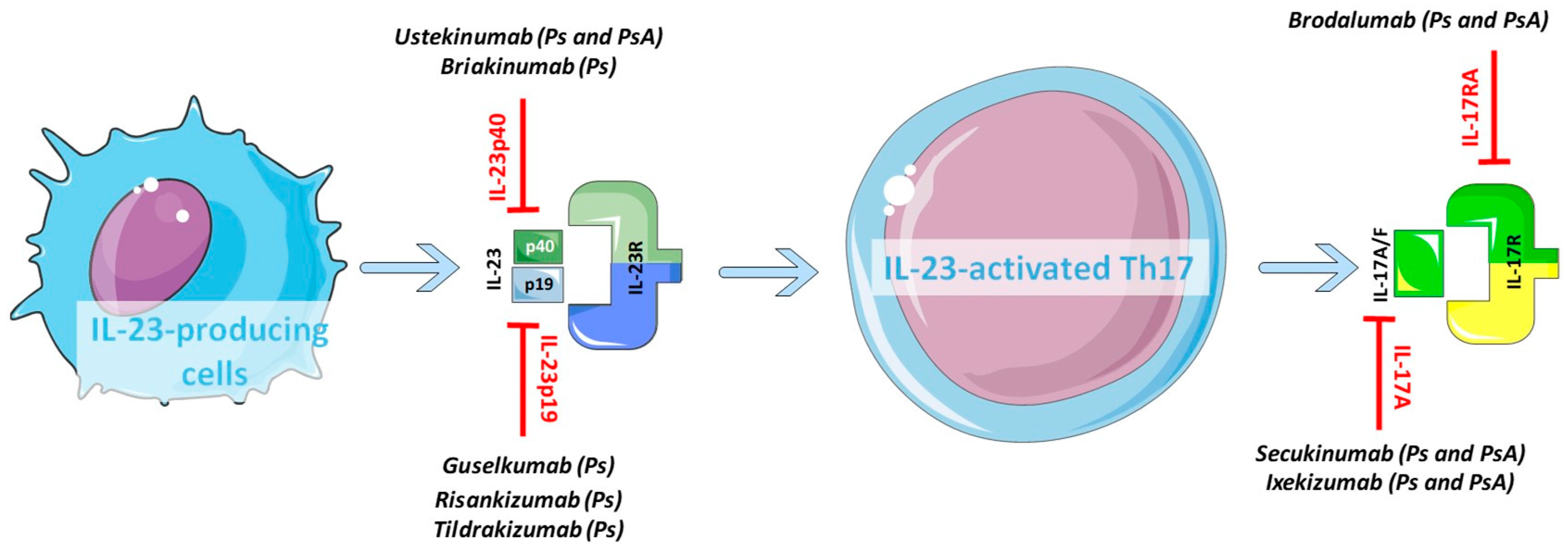
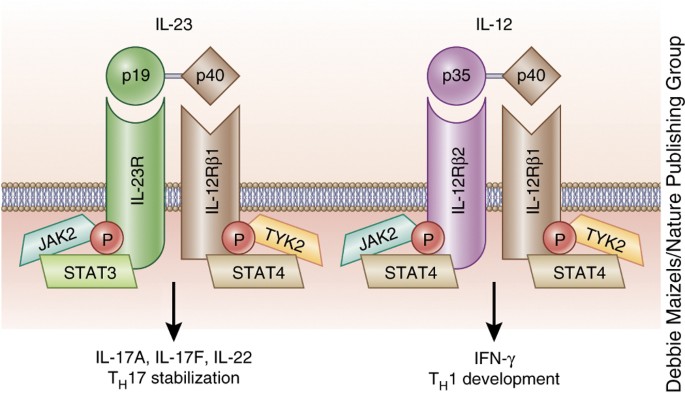
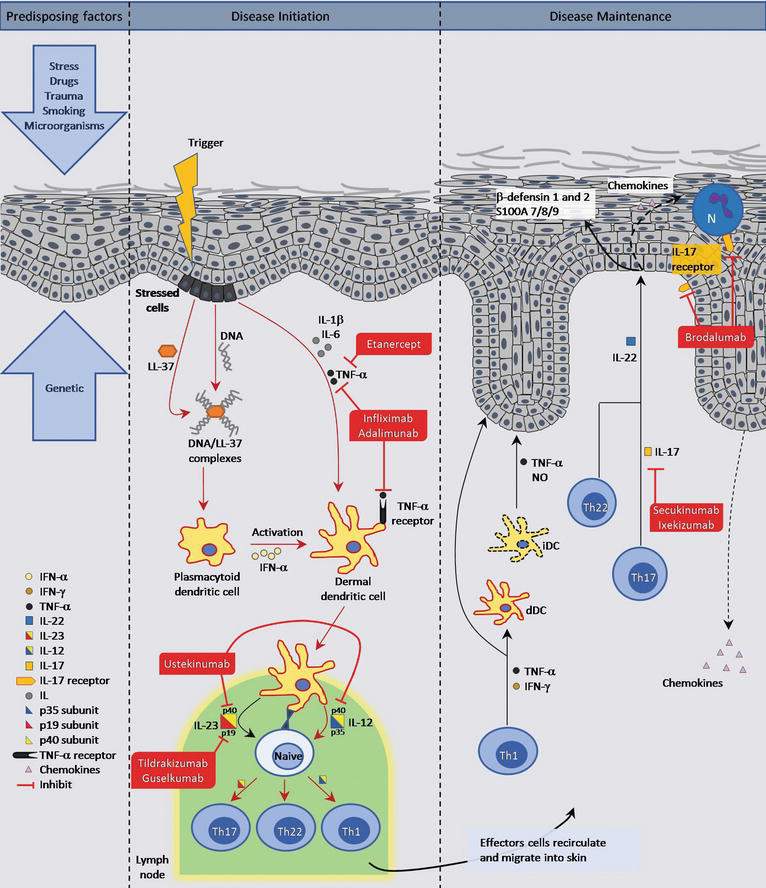
Il 23 inhibitor drugs-29/4/ Therapies such as ustekinumab and guselkumab inhibit IL23 Ustekinumab targets the p40 subunit common to both IL23 and IL12 while guselkumab targets the p19 subunit found in IL23 IL17 subtypes trigger downstream inflammation in psoriasisInterleukin23 can be targeted by using an antibody against IL12/IL23;
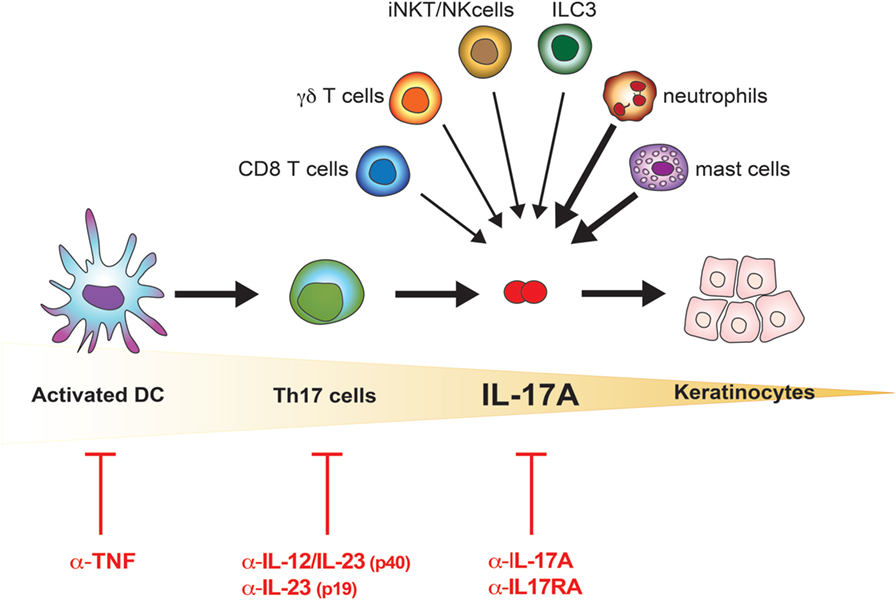



Frontiers The Il 17 Family Of Cytokines In Psoriasis Il 17a And Beyond Immunology
24/7/ UCB's IL17 latecomer bimekizumab beats Cosentyx in psoriasis trial Belgian biopharma company UCB is bringing up the rear of the IL17 inhibitor category with its bimekizumab drug, so isThe advent of monoclonal antibodies targeting tumor necrosis factor alpha (TNFα) dramatically altered the therapeutic landscape in inflammatory bowel disease (IBD) over the last years, enabling a paradigm shift away from the traditional stepcare approach to therapy focused primarily on symptom alleviation in favor of a more aggressive topdown approach more likely to induceInterleukin 17 family (IL17 family) is a family of proinflammatory cystine knot cytokines They are produced by a group of T helper cell known as T helper 17 cell in response to their stimulation with IL23Originally, Th17 was identified in 1993 by Rouvier et al who isolated IL17A transcript from a rodent Tcell hybridoma The protein encoded by IL17A is a founding member of IL17 family
The IL23 inhibitors have also been shown to lead to a long duration of remission after withdrawal of the drug In trials of TNFα inhibitors, the median time to loss of PASI 50 had a range of 121–195 weeks after discontinuation of therapy 7913/5/21 IL12 inhibitors Pipeline Analysis The "IL12 InhibitorsPipeline Insights 18″ report covers an indepth analysis of IL12 inhibitor drug molecules currently undergoing clinical studies It provides a deep understanding of potential IL12 inhibitor drug molecules across all drug development phases1/5/21 Several drugs targeting the IL23/IL17 axis have been successfully tested in PsO and Ps For example, ustekinumab and secukinumab, inhibitors of IL12/IL23p40 and IL17A respectively, are recommended as a secondline biological treatment for PsA patients inadequate responders to conventionalsynthetic (cs) diseasemodifying antirheumatic drugs (DMARDs)
6/8/ Tildrakizumab — a humanized monoclonal antibody that selectively inhibits the p19 subunit of IL23 9 — was the second IL23 antagonist to receive FDA approval for the treatment of psoriasis It was approved in 18 for the treatment of moderate to severe plaque psoriasis in adults who may benefit from systemic therapy or phototherapy23/3/21 The IL23 inhibitors include Stelara (ustekinumab), Tremfya (guselkumab), Ilumya (tildrakizumabasmn) and Skyrizi (risankizumabrzaa) while the IL17 inhibitors include Cosentyx (secukinumab), Taltz (ixekizumab) and Siliq (brodalumab)IL23 inhibitors are effective in treating psoriasis and psoriatic arthritis IL23 inhibitors are safe and do not show a significantly increased risk for adverse events



1
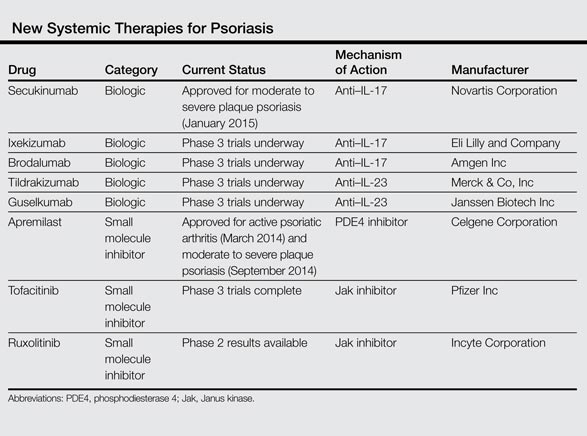



New Systemic Therapies For Psoriasis Mdedge Dermatology
22/2/18 IL18 inhibitor drug holds great potential for inflammatory diseases An international team of researchers has identified the key role that IL18 protein plays in Still's disease, making it a prime candidate for IL18 inhibitor drug that is proving promising in current trials Still's disease is a serious orphan disease characterised byInterleukin Inhibitors A recombinant form of human interleukin1 receptor antagonist used in the treatment of rheumatoid arthritis and neonatalonset multisystem inflammatory disease A monoclonal antiC25 antibody (interleukin2 receptor alpha subunit) used as immunosuppressive therapy in kidney transplant patientsInterleukin23 (IL23) Inhibitor Market Research Report 1925 by Players Regions Product Types & Applications Size, Share & Forecast to 25
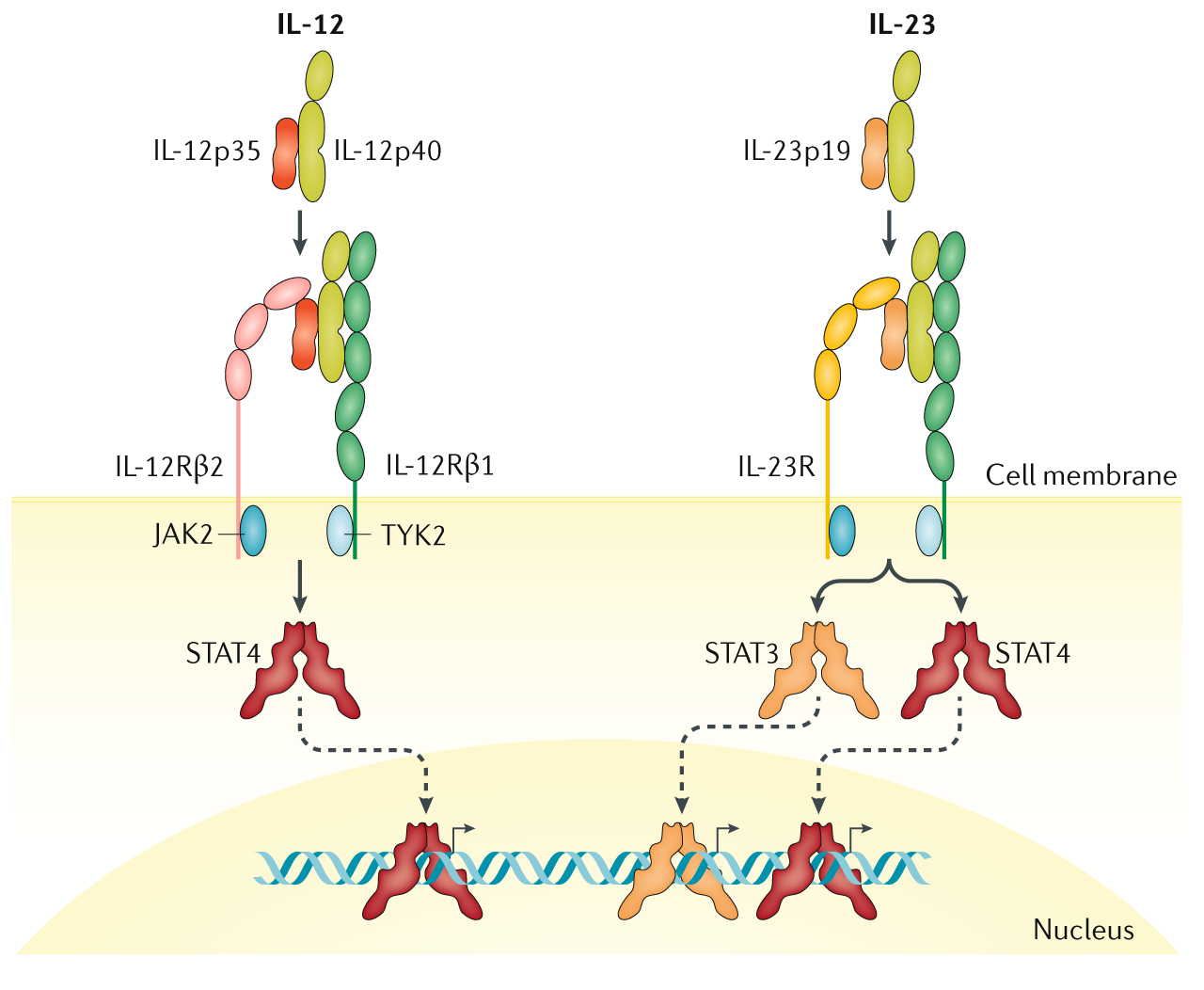



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology




Efficacy And Safety Of Ustekinumab An Il 12 And Il 23 Inhibitor In Patients With Active Systemic Lupus Erythematosus Results Of A Multicentre Double Blind Phase 2 Randomised Controlled Study The Lancet
Interleukin23 (IL23) Inhibitor Pipeline Insight, report by DelveInsight offers comprehensive insights of the pipeline (under development) therapeutics scenario and growth prospects across Interleukin23 (IL23) Inhibitor development7/2/21 The ECLIPSE clinical trial was the very first study to investigate guselkumab (an IL23 inhibitor) side by side secukinumab (an IL17 inhibitor) 84% of those taking guselkumab reached the trial's primary endpoint Only 70% of those taking secukinumab reached the same endpoint Each drugs' previous safety profile was maintainedJuly 14, Accessed July 14,HealthDay News –7/5/ Interleukin23 (IL23) inhibitors are an important new class of drugs for the treatment of Crohn disease (CD) and ulcerative colitis (UC), both common causes of inflammation of the digestive tract Johnson & Johnson's Stelara (ustekinumab) is the only IL23 inhibitor currently approved to treat moderatetosevere CD and UC in the United States




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect




Critical Role Of Interleukin Il 17 In Inflammatory And Immune Disorders An Updated Review Of The Evidence Focusing In Controversies Sciencedirect
1/2/15 Interleukin17 (IL17) is believed to be a potent driver of plaque psoriasis This article reviews efficacy and safety results from Phase 2 and 3 trials with monoclonal antibodies targeting IL17RA (brodalumab), and IL17A (ixekizumab and17/9/19 The efficacy of IL23 inhibitors is yet to be determined in both treating psoriatic arthritis and reducing systemic inflammation Because there are few contraindications to the use of IL23 inhibitors, patients with IBD, multiple sclerosis, liver disease, or heart disease may all be considered for these drugs25/4/16 IL12, IL23 Inhibitor More Effective Than TNF Inhibitors in Psoriasis HealthDay News – Ustekinumab was more effective than tumor necrosis factorα inhibitors for the treatment of psoriasis at 6 and 12 months, according to a study published in the May issue of the Journal of the American Academy of Dermatology
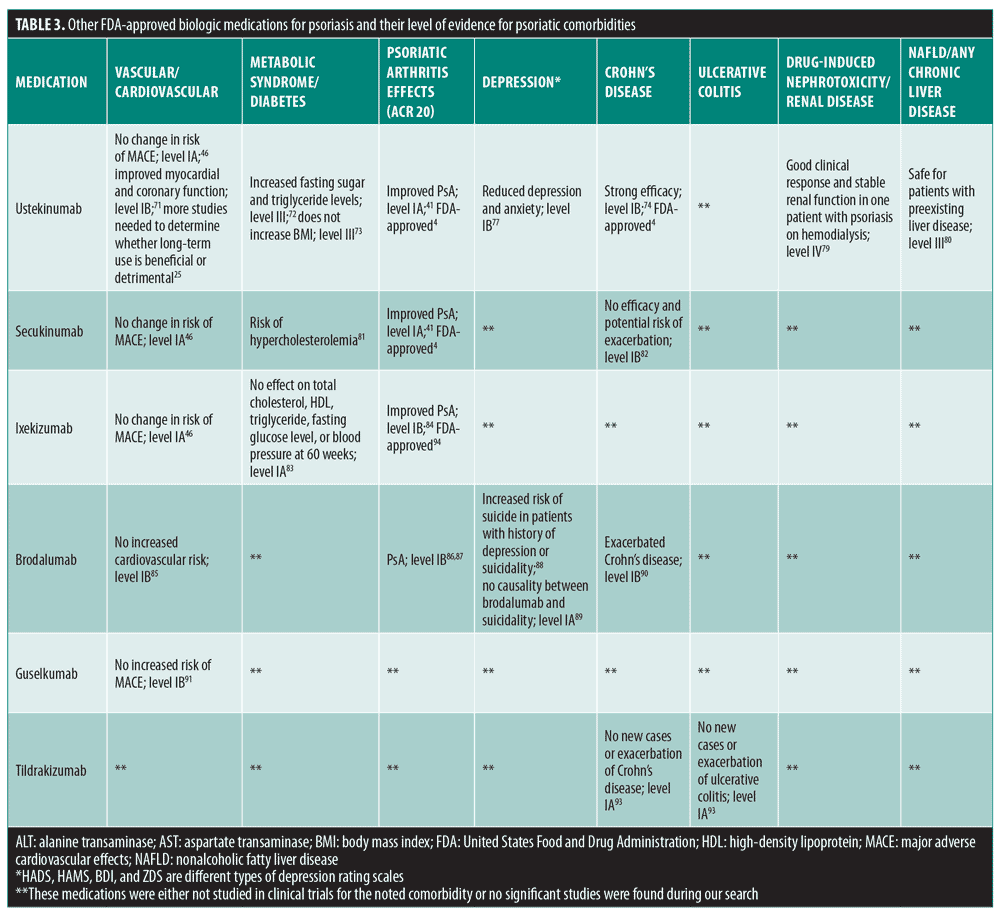



Systemic Psoriasis Therapies And Comorbid Disease In Patients With Psoriasis A Review Of Potential Risks And Benefits Jcad The Journal Of Clinical And Aesthetic Dermatology




Induction Therapy With The Selective Interleukin 23 Inhibitor Risankizumab In Patients With Moderate To Severe Crohn S Disease A Randomised Double Blind Placebo Controlled Phase 2 Study The Lancet
13/3/ IL17 inhibitors secukinumab (Cosentyx), ixekizumab (Taltz), and brodalumab (Siliq) IL12/IL23 inhibitor ustekinumab (Stelara) Doctors may also prescribe one or more of the following treatmentsNevertheless, it would be much more useful to design drugs that target the IL23p19 or IL23 receptor, so inhibiting IL23 without modifying the effects of IL12Secukinumab had the lowest (799%) At 24 months, guselkumab had the highest cumulative probability of



Evolution Of Treatment Strategies Targeting Il 23 For Psoriasis Ppt Download



Il Inhibitors Market Size Share Industry Trends Report 19 26
1/9/18 Thus, biologics targeting IL23 might be a better choice for patients with psoriasis in terms of their drug adherence and safety A recent metaanalysis performed by Bilal et al also evaluated 24 trials of IL17 and IL23 pathway inhibitors for moderate to severe plaque psoriasisApilimod is a small molecule that inhibits IL12 and IL23 production cytokines that are involved in autoimmune diseases through the prevention of nuclear translocation of cRel Synta Pharmaceuticals Corp is developing apilimod for the potential treatment of Crohn's disease (CD) and other autoimmune diseasesThe p40 subunit that is shared by IL12 and IL23, was approved for psoriasis in 09 and psoriatic arthritis in 13, and there are several antibodies in development that target the IL23specific p19 subunit, with a more specific effect on the IL17 pathway Drugs targeting the IL17 pathway are now being tested in a growing list of indications




Efficacy And Safety Of Biologics Targeting Il 17 And Il 23 In The Treatment Of Moderate To Severe Plaque Psoriasis A Systematic Review And Meta Analysis Of Randomized Controlled Trials Sciencedirect



Auto Immune Arthropathy Understanding The Il 23 Il 17 Pathway Gp Voice
10/2/ Ustekinumab (Stelara) is an IL12/23 inhibitor that's FDpproved to treat psoriasis IL23 inhibitors These inhibitors can then effectively blockRisankizumab, guselkumab, and tildrakizumab are new IL23 inhibitors currently in phase 3 trials with promising early efficacy and safety results Ixekizumab, which recently was approved, and brodalumab, which is pending US Food and Drug Administration review, are new IL17 inhibitors that achieved total skin clearance in more than onequarter of phase 3 participants after 12BS A handful of agents have been, or are currently being, looked at as inhibitors of either interleukin (IL)12 and 23 or just IL23 Ustekinumab (Stelara, Janssen), which was recently approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe Crohn's disease, is an antibody directed against the p40 subunit shared by IL12 and 23




Scielo Brasil Biologic Therapy For Psoriasis Still Searching For The Best Target Biologic Therapy For Psoriasis Still Searching For The Best Target



Cells Free Full Text Decoding Il 23 Signaling Cascade For New Therapeutic Opportunities
Key players involved in Interleukin23 (IL23) Inhibitor targeted therapeutics development with respective active and inactive (dormant or discontinued) projects Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular typeIl6 is one another Can we inhibit il23 My father is suffering from Pulmonary fibrosis currently he is taking Pirfenidone which is the recent drug Interleukin23 inhibitorsArticles 1330 wwwthelancetcom Vol 392 Efficacy and safety of ustekinumab, an IL12 and IL23 inhibitor, in patients with active systemic lupus erythematosus results of a multicentre, doubleblind,




Frontiers The Il 17 Family Of Cytokines In Psoriasis Il 17a And Beyond Immunology




Interleukin 23 As A Drug Target For Autoimmune Inflammatory Diseases Tang 12 Immunology Wiley Online Library
LAS VEGASâ Two approved and two pending antiIL 23 drugs offer an opportunity for moderate to severe psoriasis patients to regain their lives, according to a presentation at the 18 Fall Clinical Dermatology ConferenceThe Interleukin23 (IL23) Inhibitor report provides an overview of therapeutic pipeline activity and therapeutic assessment of the products by development stage, product type, route of administration, molecule type, and MOA the complete product development cycle, including all clinical and nonclinical stages7/8/18 ICER IL23 Inhibitors Are Preferable to AntiTNF Agents for Plaque Psoriasis Samantha DiGrande The Institute for Clinical and Economic Review (ICER) explains that, compared with anti–tumor necrosis factor (antiTNF) drugs, both guselkumab and risankizumab offered a superior benefit based on currently available data



Ijms Free Full Text Role Of The Il 23 Il 17 Axis In Psoriasis And Psoriatic Arthritis The Clinical Importance Of Its Divergence In Skin And Joints Html
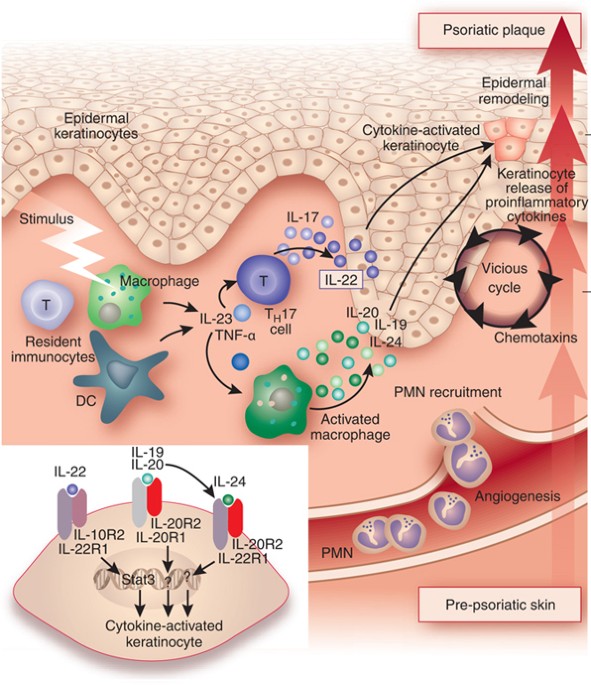



New Anti Il 23 Drugs Raise Hopes For Psoriasis Plaque Clearance Nature Biotechnology
10/9/19 Background The role of interleukin12 (IL12), interleukin23 (IL23), and interleukin17 (IL17) has been recognized in psoriasis pathogenesis, and new drugs targeting this axis have already been developed which may provide a new therapeutic approach for patients with moderate to severe psoriasis Objective To compare the direct and indirect evidences of the efficacy and5/6/17 Interleukin 23 is a heterodimeric cytokine composed of two subunits p40, which is also a subunit of interleukin 12 and is targeted by ustekinumab (a biological drug approved for psoriasis and psoriatic arthritis), and p19, which is expressed in interleukin 23 only Likewise, the development of specific inhibitors of interleukin 23 followed the22/5/19 Latecomer Lilly preps its IL23 drug for Crohn's disease Hopes to leapfrog rivals Eli Lilly's IL23 inhibitor mirikizumab is trailing the field in lead indication psoriasis – where it looks set to be fifth to market – but could leapfrog some of its rivals in Crohn's disease




Psoriasis And Psa Beyond Skin And Joint Involvement




Il 23 Inhibitors For Treating Psoriasis What To Know
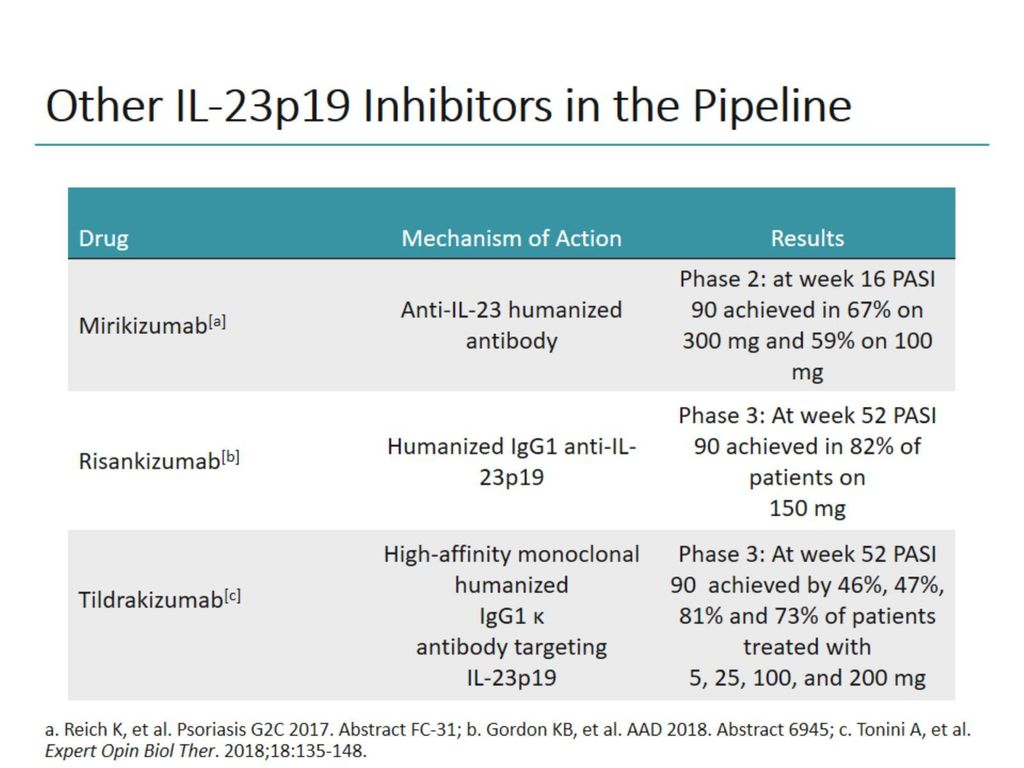
At this time, 2 inhibitors of IL23 p19 have been approved by the United States Food and Drug Administration, guselkumab and tildrakizumab Two other agents, risankizumab and mirikizumab, have completed phase 3 and phase 2 of development, respectively They suggest that people whose conditions do not respond to their first TNF inhibitor should try another TNF inhibitor rather than using IL17 Three p19based IL23 inhibitors are currently approved for psoriasis guselkumab (first approved), tildrakizumab, and risankizumab (most recently approved) This study assessed the relative benefits and risks of these drugs using adalimumab as a common comparator
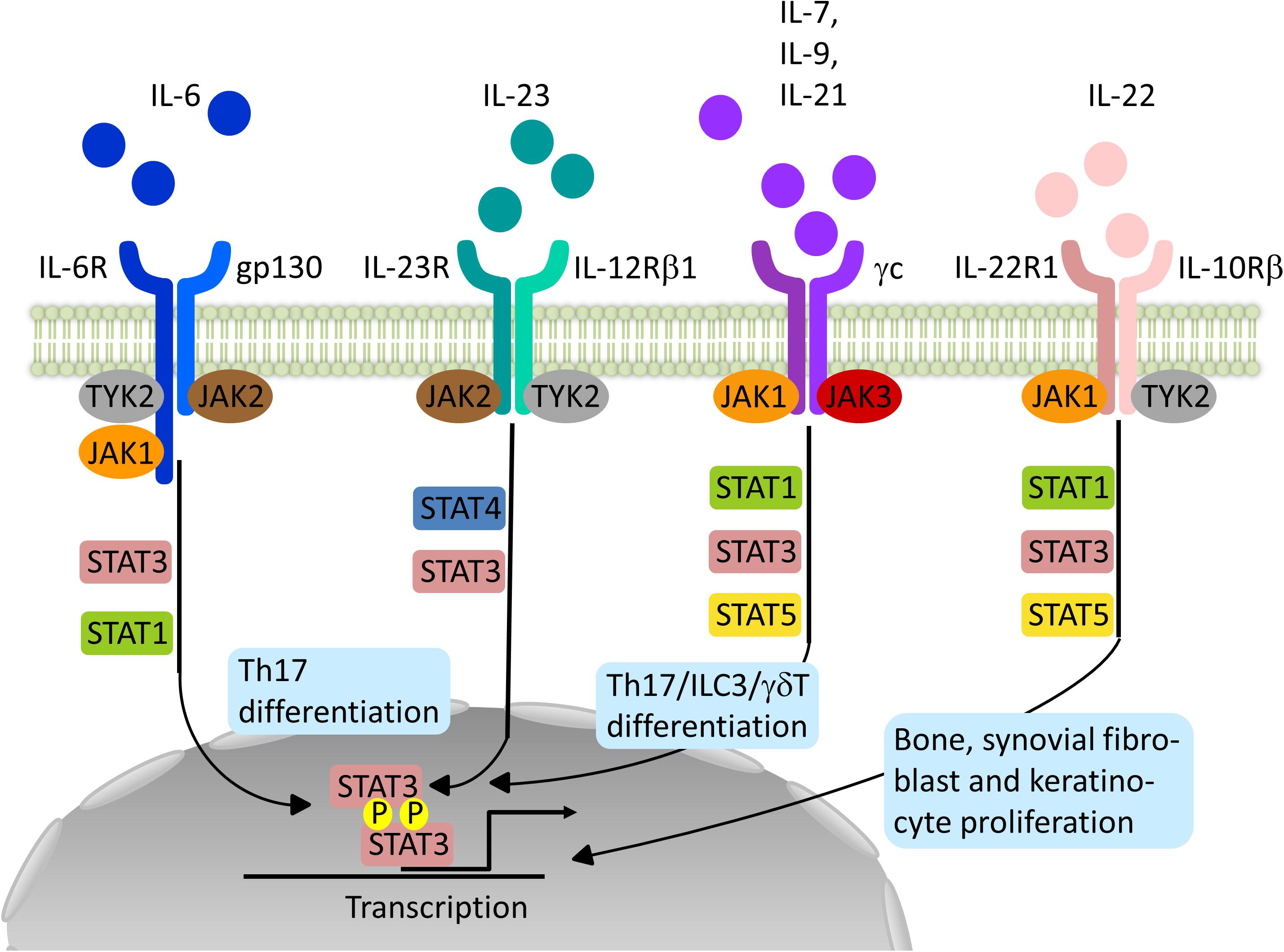



Frontiers Impact Of Janus Kinase Inhibition On The Treatment Of Axial Spondyloarthropathies Immunology
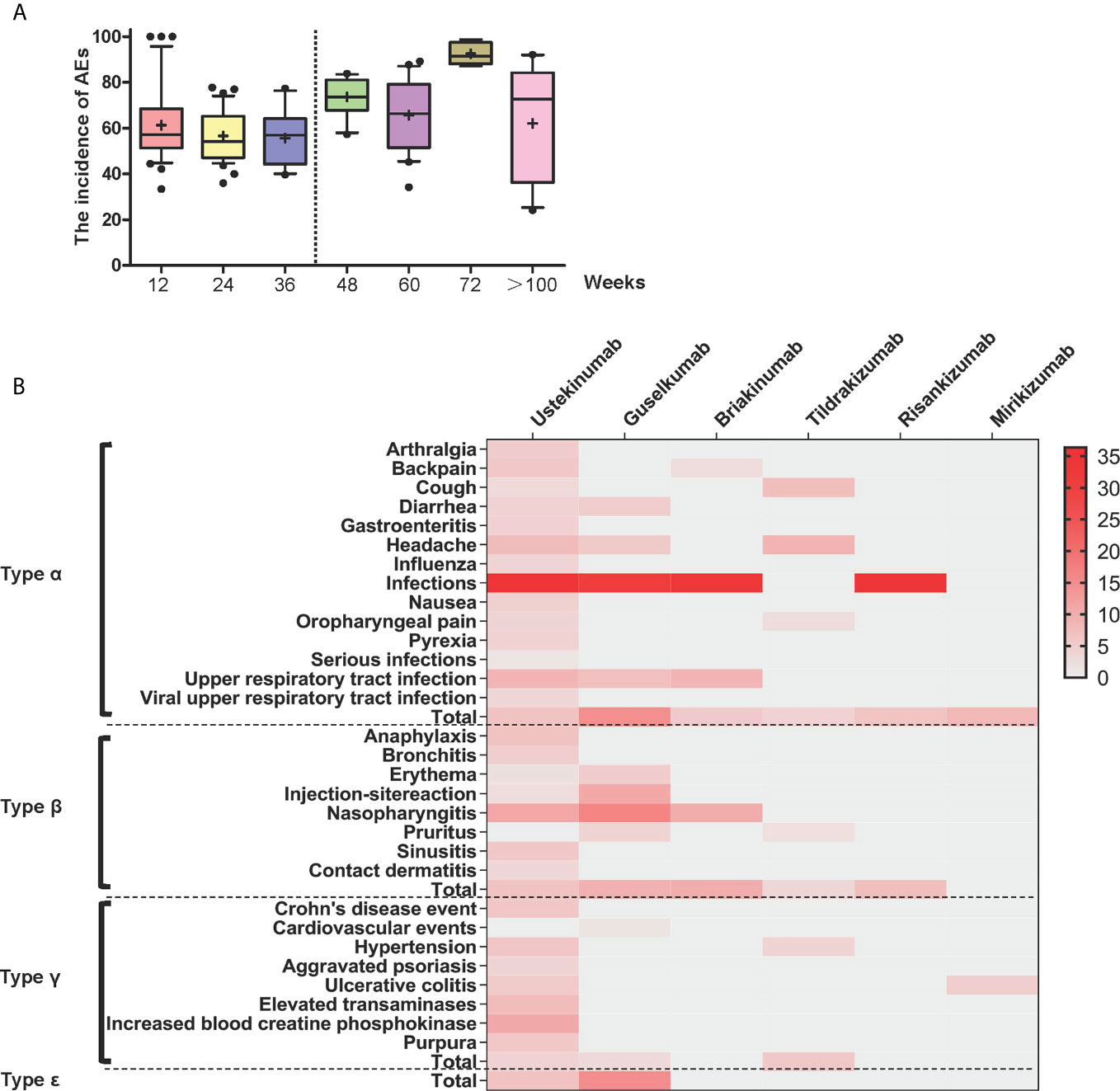



Frontiers Adverse Events Associated With Anti Il 23 Agents Clinical Evidence And Possible Mechanisms Immunology
Introduction Blockade of interleukin (IL)12 and IL23 is a novel therapeutic target for inflammatory bowel disease (IBD) The monoclonal antibody targeting the shared p40 subunit of IL12 and IL23, namely ustekinumab, has been approved for Crohn's disease (CD) and has demonstrated promising results in the treatment of ulcerative colitis Supported By EU CHMP, AbbVie IL23 Inhibitor Is Expected To Gain The Second Indication On , AbbVie announced that the European Medicines Agency (EMA) Committee for Medicines for Human Use (CHMP) recommended to approve risankizumab (trade name Skyrizi) as a single drug or in combination with methotrexate (MTX)21/1/21 The results of several clinical trials have shown novel biologic agents such as ixekizumaband guselkumab to be highly effective for patients with moderate to severe psoriasis 69 Recently approved by the FDA as the first selective IL23 inhibitor for the treatment of psoriatic arthritis, guselkumab is a human immunoglobulin G1λ monoclonal antibody targeting the p19




Psoriasis Treatment Unmet Needs Present 19 Opportunities Pm360




New Mechanisms And Expanded Indications For Biologic Therapies A Perspective On Immunology Research And Development Drug Discovery World Ddw




Interleukin 23 In Psoriasis Integrating New Therapies In The Current Treatment Landscape European Medical Journal
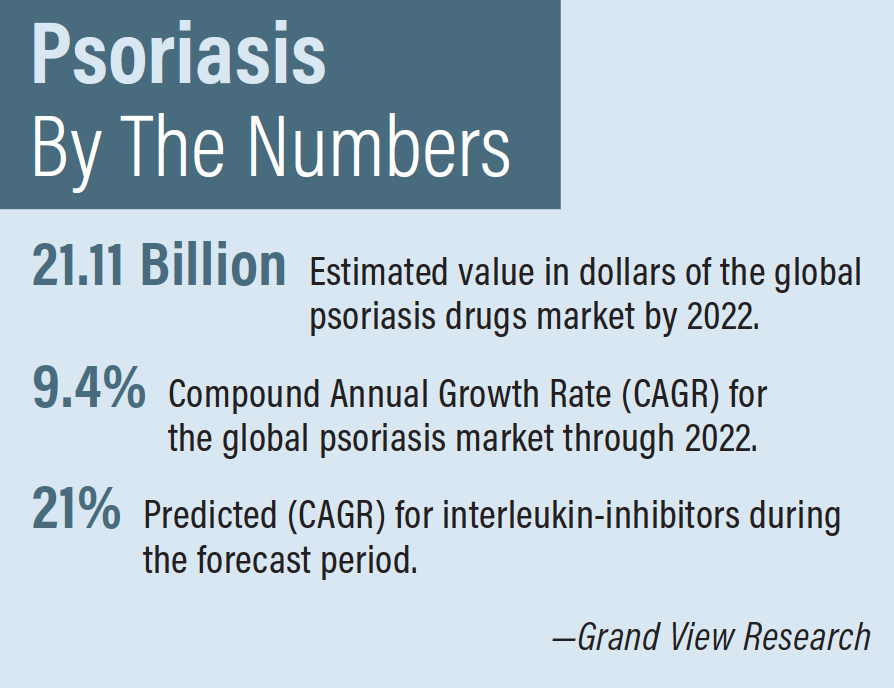



A Closer Look At Skyrizi Practical Dermatology




Interleukin 17 Wikipedia
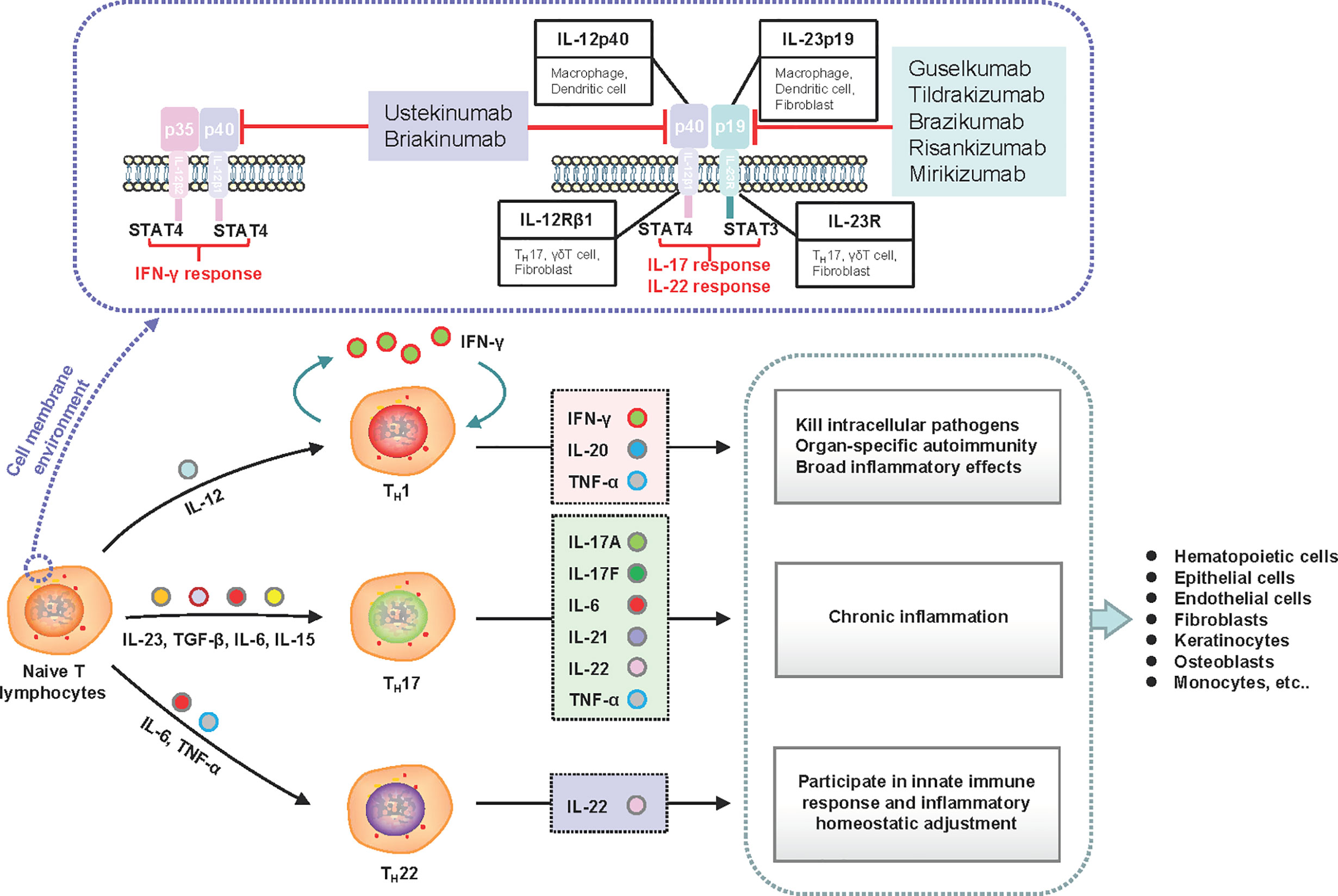



Frontiers Adverse Events Associated With Anti Il 23 Agents Clinical Evidence And Possible Mechanisms Immunology




American Journal Of Clinical Dermatology Drug Survival Of Il 12 23 Il 17 And Il 23 Inhibitors For Psoriasis Treatment A Retrospective Multi Country Multicentric Cohort Study In This Multinational Cohort With 8439 Patient Years Of Follow Up
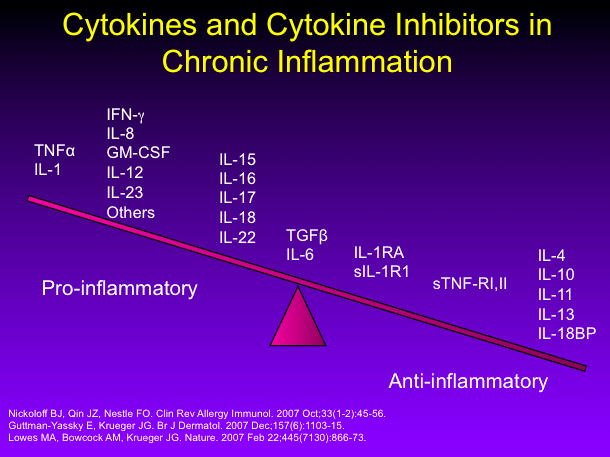



Systemic Treatment Of Psoriasis What S New Maui Derm




Systematic Review Of Interleukin 12 Interleukin 17 And Interleukin 23 Pathway Inhibitors For The Treatment Of Moderate To Severe Chronic Plaque Psoriasis Semantic Scholar
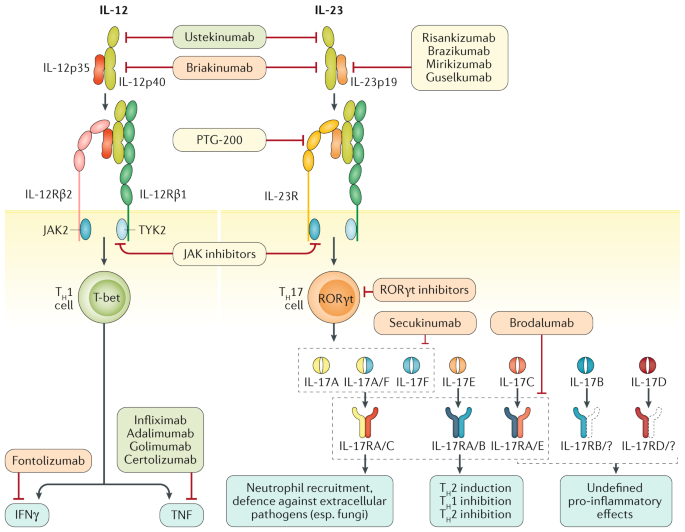



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology
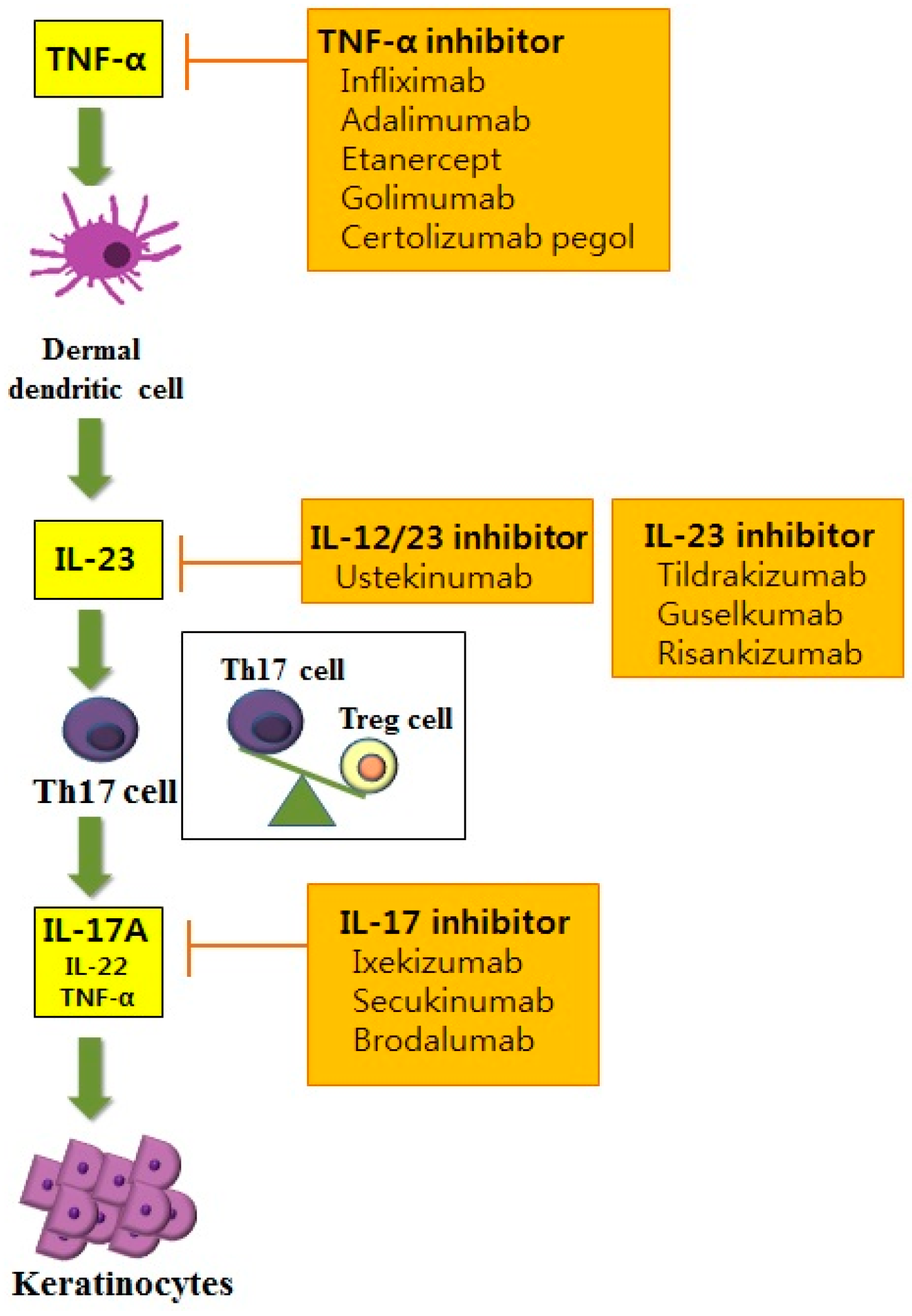



Ijms Free Full Text Molecular Mechanisms And Management Of A Cutaneous Inflammatory Disorder Psoriasis Html
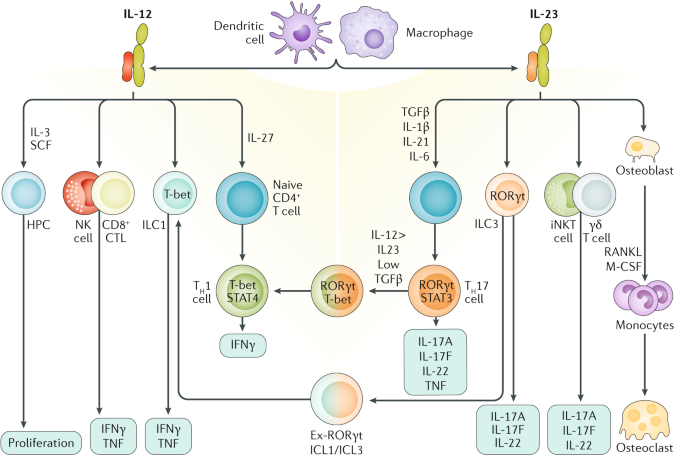



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology




Pdf Monograph Advances In Psoriasis Semantic Scholar




New Biologics And Small Molecules Under Development For The Treatment Download Scientific Diagram




Il 23 Antagonists In The Treatment Of Plaque Psoriasis Dermatology Advisor
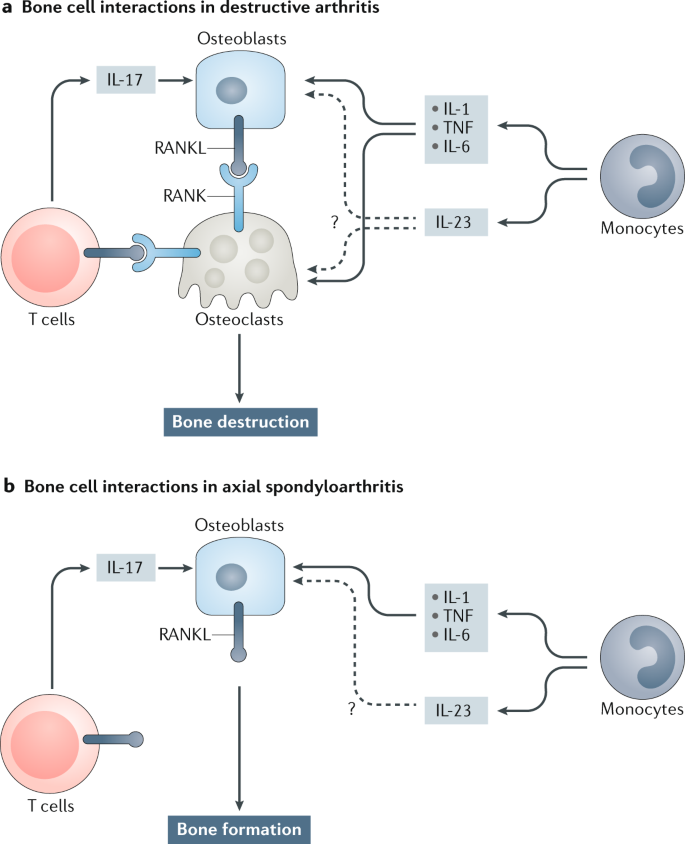



The Il 23 Il 17 Pathway As A Therapeutic Target In Axial Spondyloarthritis Nature Reviews Rheumatology
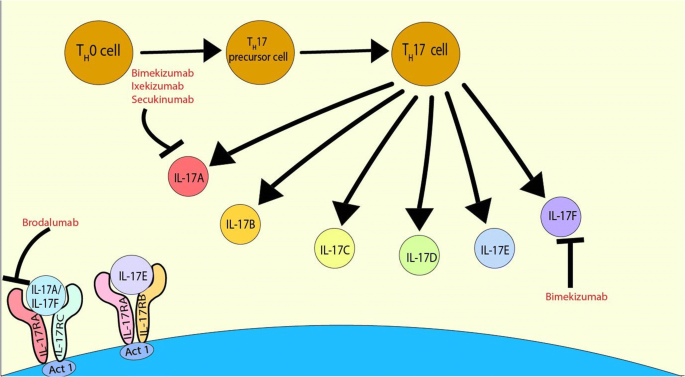



Update On Il 17 Inhibitors For Psoriasis Springerlink




Psoriatic Arthritis Treatment Toolbox Download Scientific Diagram
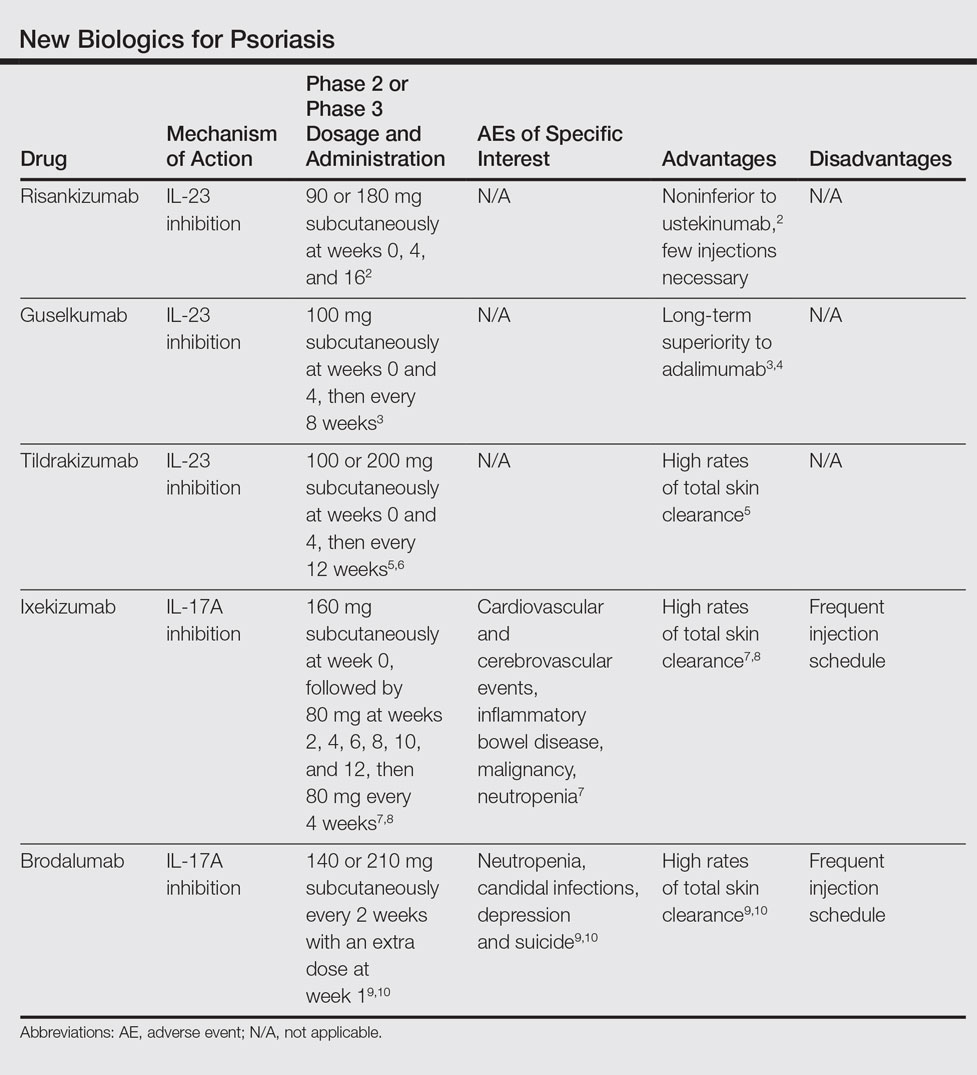



New Biologics In Psoriasis An Update On Il 23 And Il 17 Inhibitors Mdedge Dermatology




Interleukin 23 Wikipedia
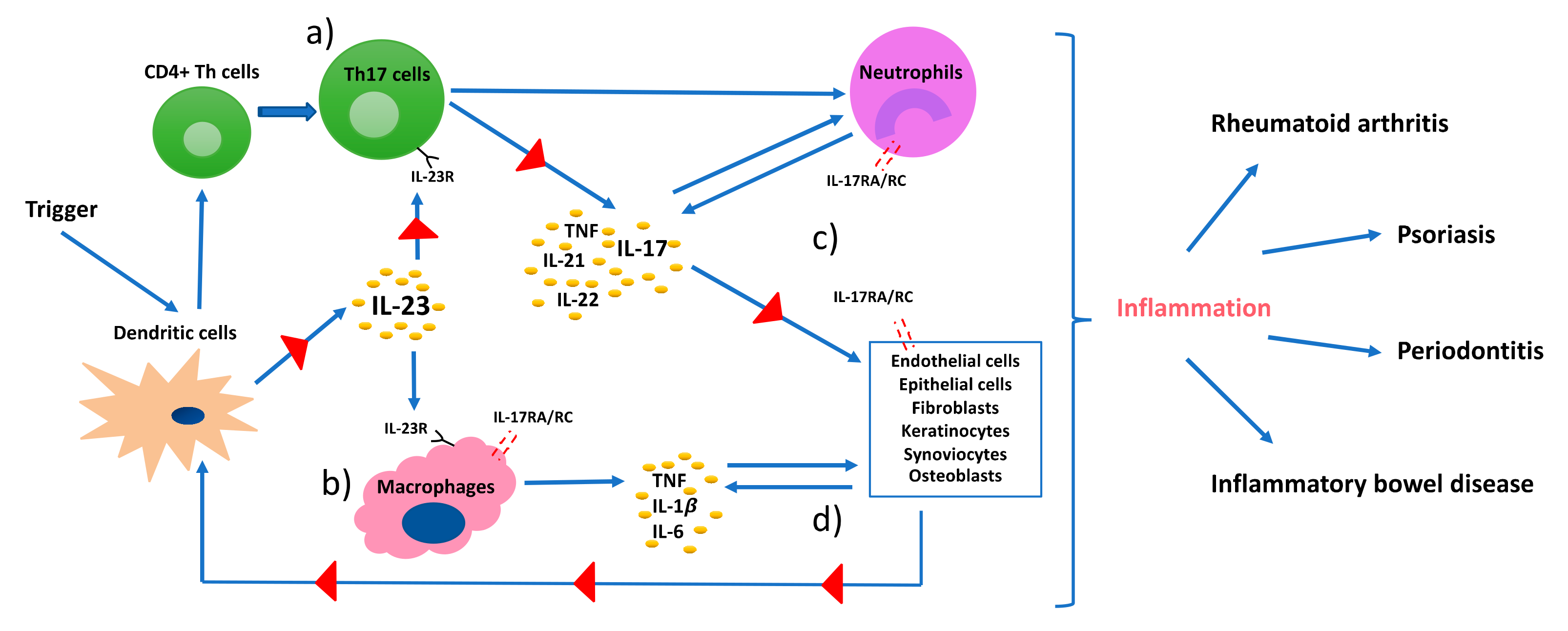



Ijms Free Full Text Th17 Cells And The Il 23 Il 17 Axis In The Pathogenesis Of Periodontitis And Immune Mediated Inflammatory Diseases Html
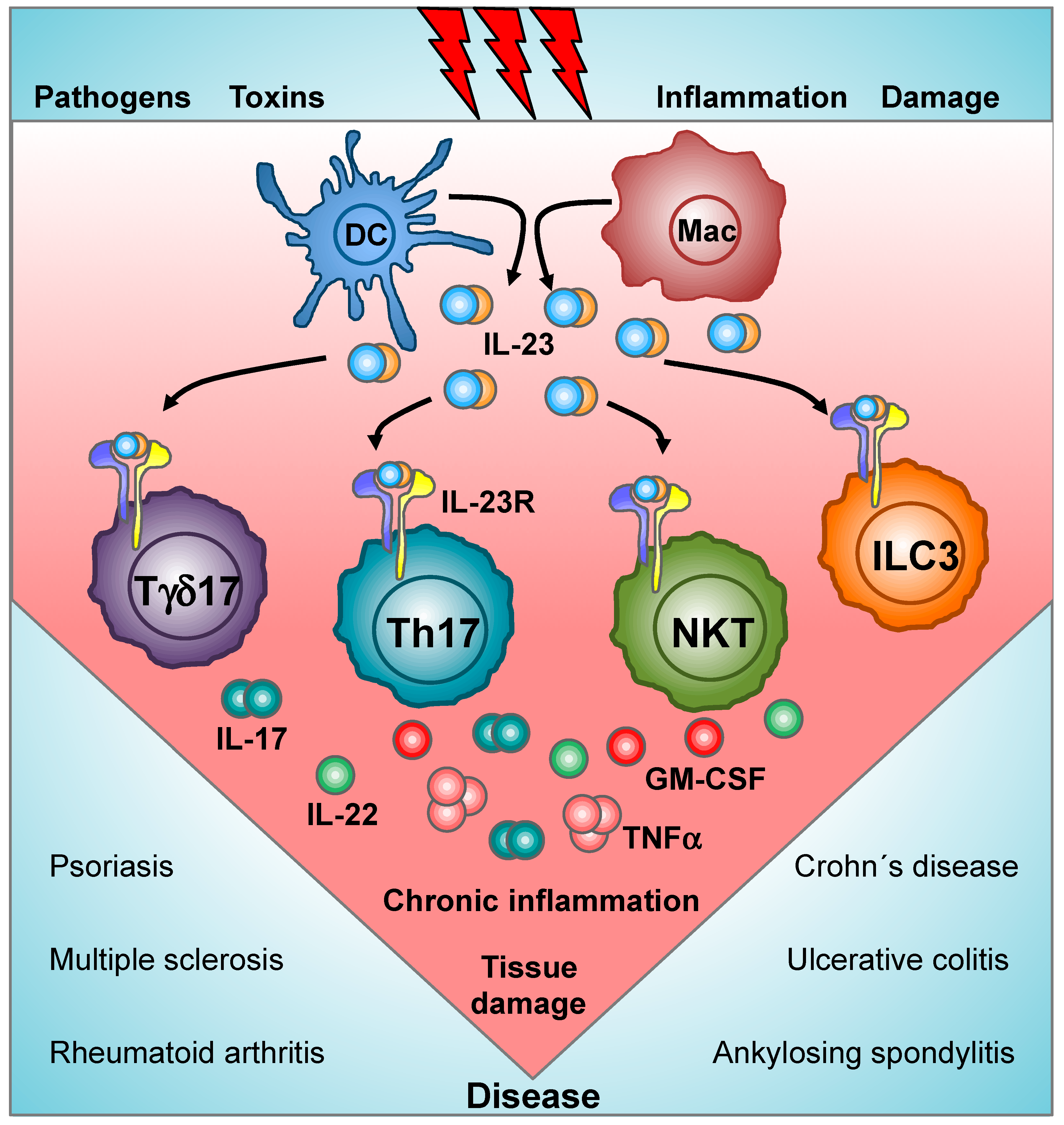



Cells Free Full Text Decoding Il 23 Signaling Cascade For New Therapeutic Opportunities




Biologic Therapies For Psoriasis Plastic Surgery Key




Lilly S Il 23 Drug Beats Novartis Cosentyx In Plaque Psoriasis Fiercebiotech
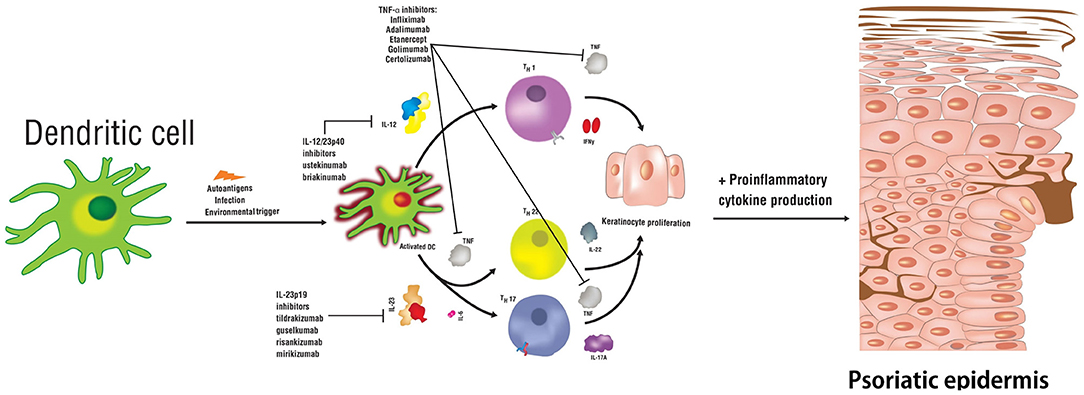



Frontiers A Review Of The Efficacy And Safety For Biologic Agents Targeting Il 23 In Treating Psoriasis With The Focus On Tildrakizumab Medicine



1




Therapeutics Targeting The Il 23 And Il 17 Pathway In Psoriasis The Lancet




Fda Approval Biological Drugs For Psoriasis Download Table



Plaque Psoriasis
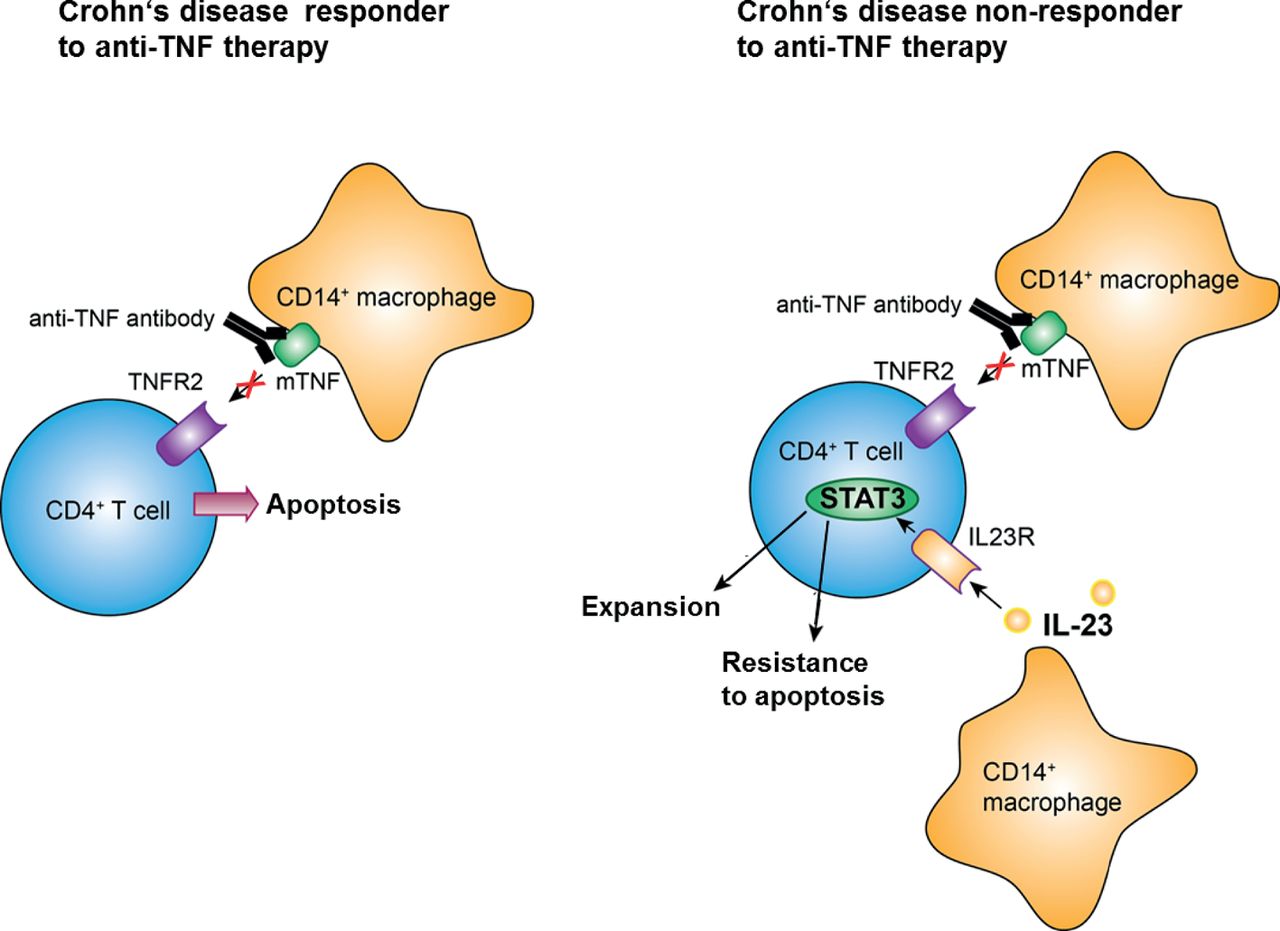



Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut




Human Th17 Cell Differentiation And Drugs Targeting This Pathway Shown Download Scientific Diagram



Il 23 Inhibitors For Treating Psoriasis What To Know




New Biologics In Psoriasis An Update On Il 23 And Il 17 Inhibitors Mdedge Dermatology




Novel Il 23 Targeted Inhibitors For Psoriasis Treatment Ppt Download
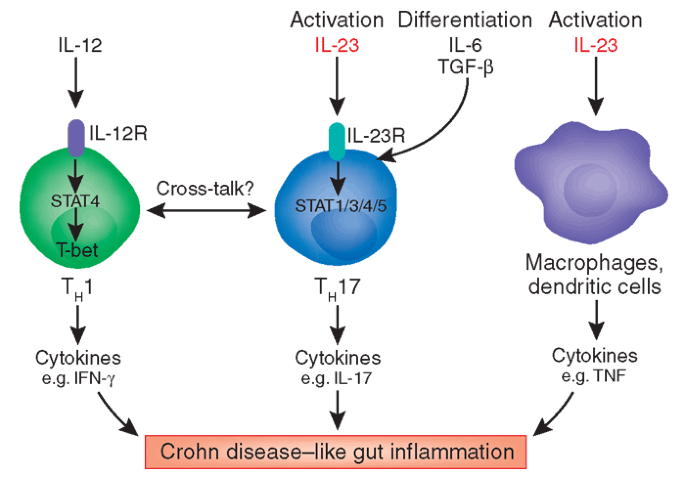



Il 23 A Master Regulator In Crohn Disease Nature Medicine




Interleukin 23 As A Drug Target For Autoimmune Inflammatory Diseases Tang 12 Immunology Wiley Online Library



New Developments In The Treatment Of Moderate To Severe Psoriasis Biologics Maui Derm




Pharmacological Inhibition Of Il 23 In Association With Adt Delays Download Scientific Diagram
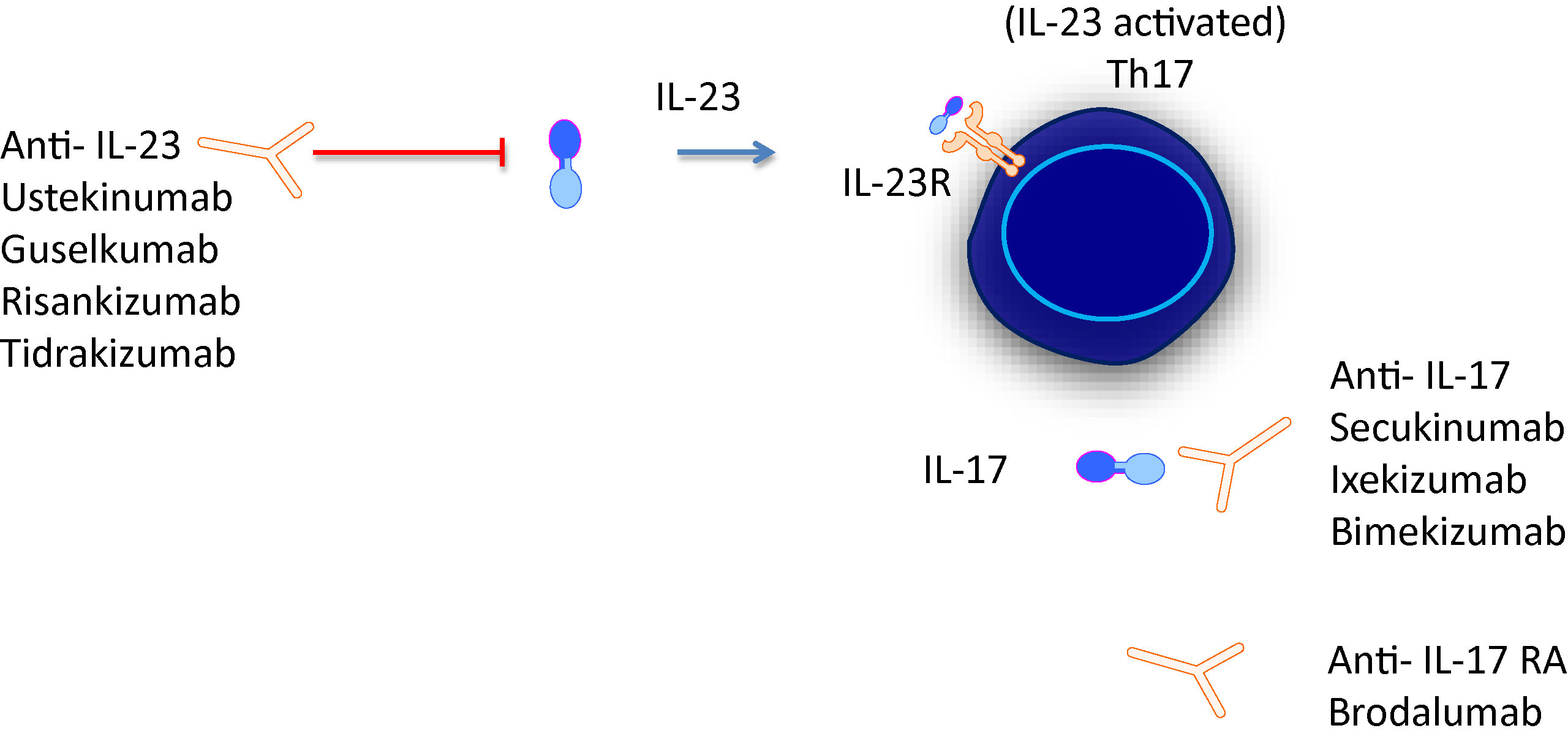



Frontiers Mini Review New Treatments In Psoriatic Arthritis Focus On The Il 23 17 Axis Pharmacology




Applying Science To Improve The Individualized Treatment Of Patients With Psoriasis Abrar Qureshi Md Mph Chief Of The Department Of Dermatology Rhode Ppt Download




Phase 3 Tremfya Results Are Promising For Plaque Psoriasis And Psoriatic Arthritis Drug Discovery And Development




Il 23 Long Term Data And Safety Data
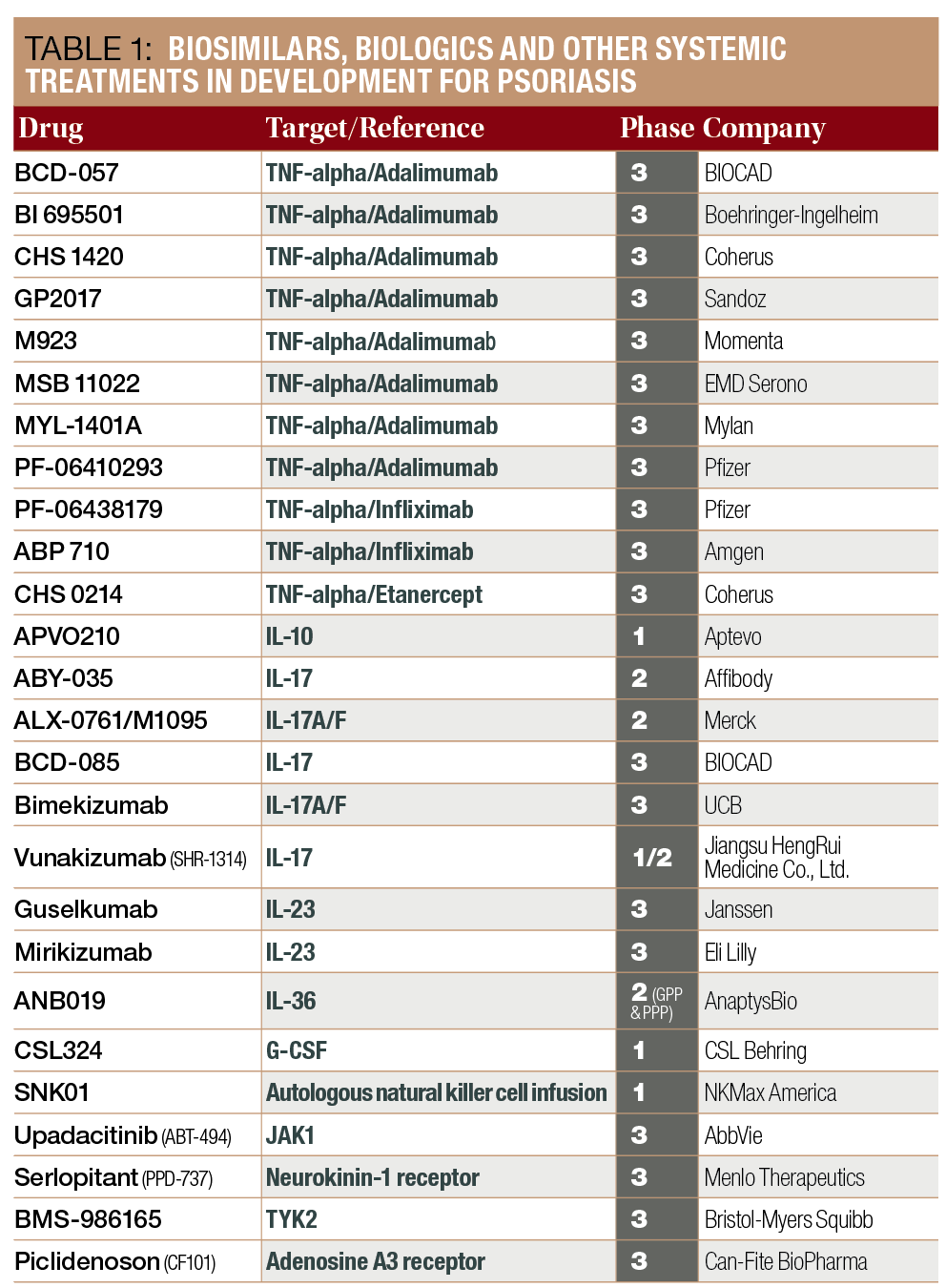



Biologics Make Psoriasis Clearance A Real Possibility



Il 12 And Il 23 Cytokines From Discovery To Targeted Therapies For Immune Mediated Inflammatory Diseases Nature Medicine
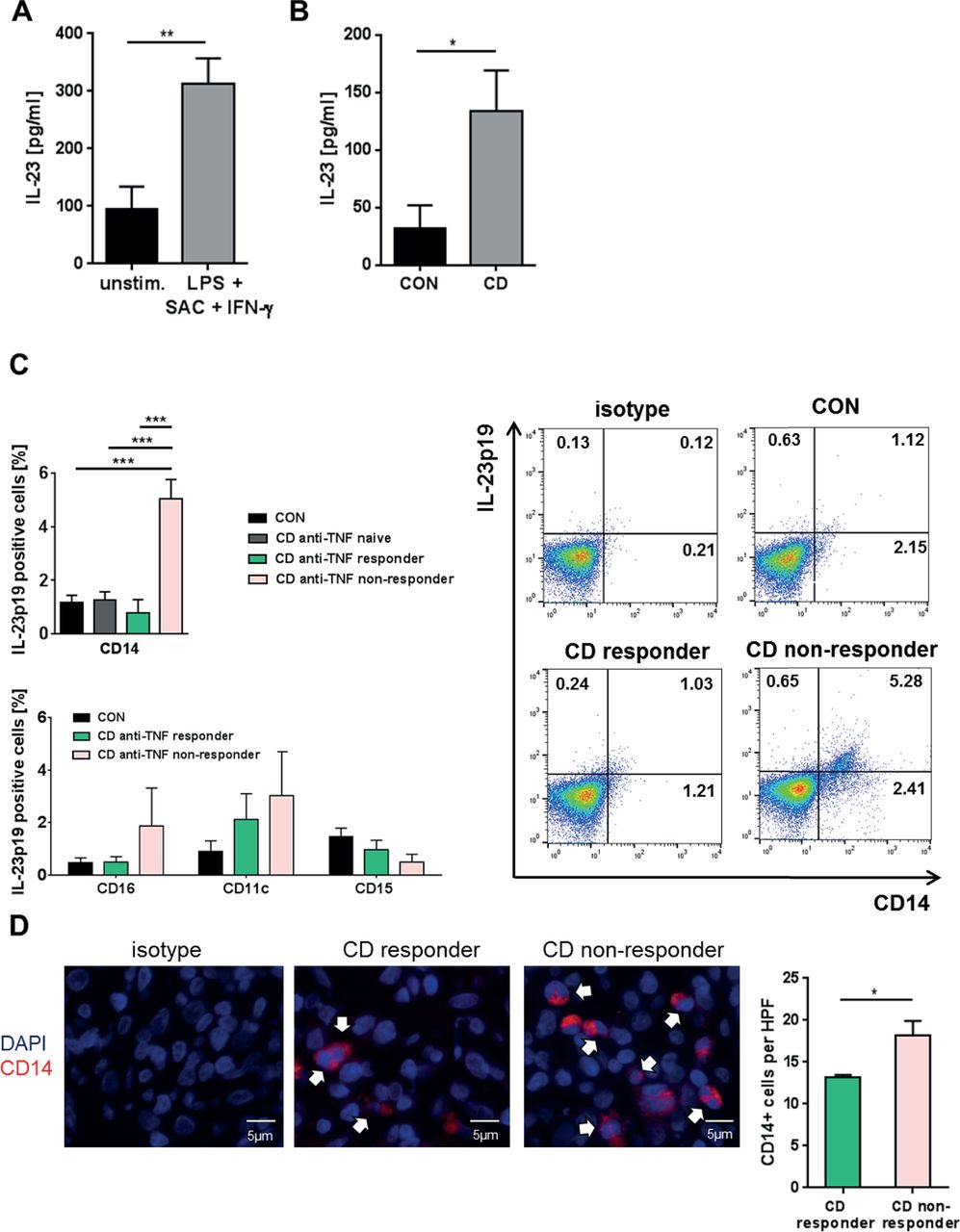



Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut
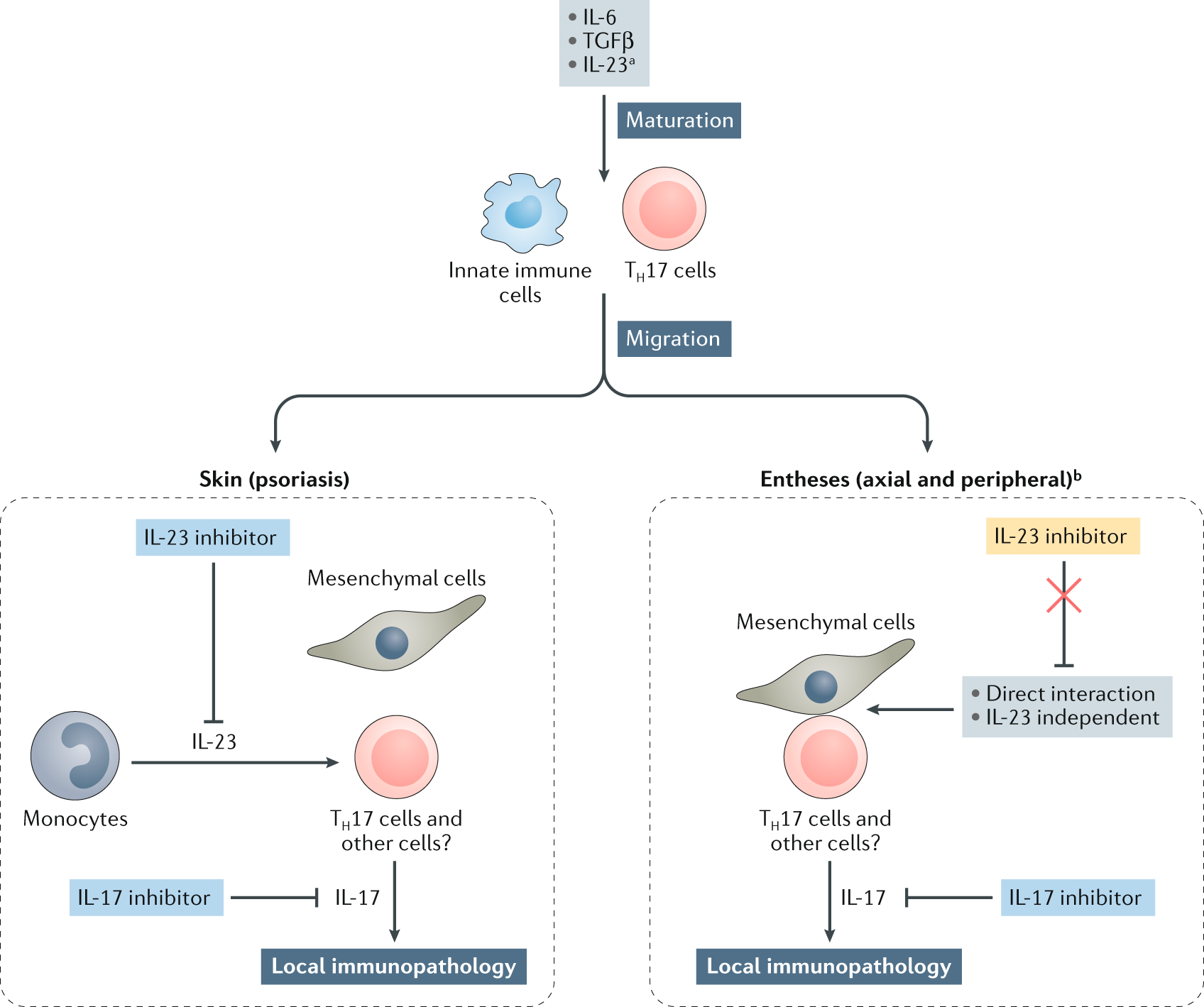



The Il 23 Il 17 Pathway As A Therapeutic Target In Axial Spondyloarthritis Nature Reviews Rheumatology




Mechanism Of Action Moa Ilumya Tildrakizumab Asmn Hcp




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect




Il 23 Inhibitors For Psoriasis Therapeutic Cheat Sheet Next Steps In Dermatology




Il 23 Inhibition From Pathophysiological Jungle To Clinical Clearance European Medical Journal




Updates In Psoriasis Management 21 Jcad The Journal Of Clinical And Aesthetic Dermatology




Il 23 Antagonists In The Treatment Of Plaque Psoriasis Dermatology Advisor




Safety Of Selective Il 23p19 Inhibitors For The Treatment Of Psoriasis Crowley 19 Journal Of The European Academy Of Dermatology And Venereology Wiley Online Library



2




Risankizumab An Il 23 Inhibitor For Ankylosing Spondylitis Results Of A Randomised Double Blind Placebo Controlled Proof Of Concept Dose Finding Phase 2 Study Annals Of The Rheumatic Diseases




Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology



The Psoriasis Drugs Market 30




New Treatments For Atopic Dermatitis Targeting Beyond Il 4 Il 13 Cytokines Annals Of Allergy Asthma Immunology




Jci Tyk2 Inhibition Reduces Type 3 Immunity And Modifies Disease Progression In Murine Spondyloarthritis




New Research Into Psoriatic Arthritis



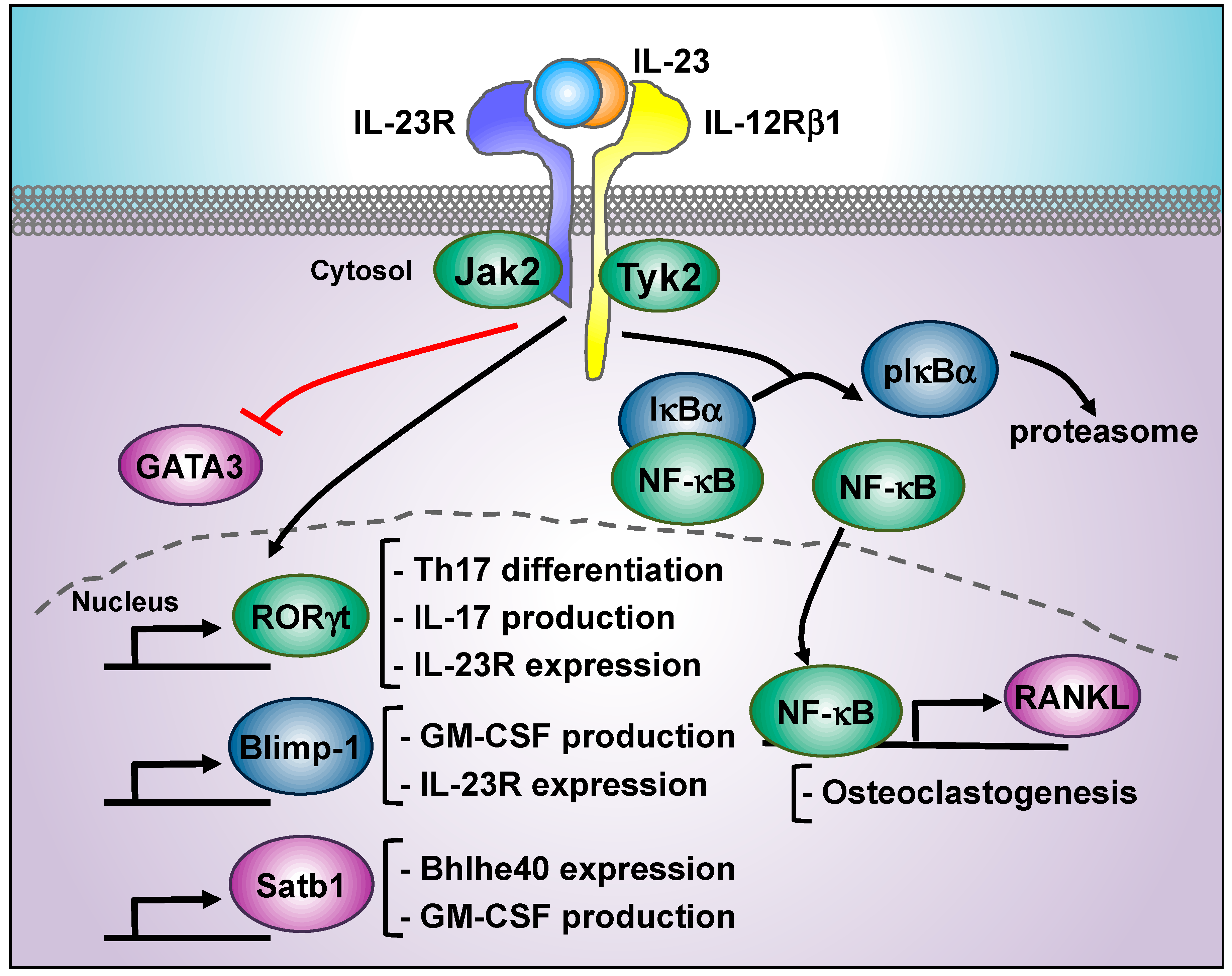
Il 23 Signaling Regulation Of Pro Inflammatory T Cell Migration Uncovered By Phosphoproteomics



Janssen S Stelara Becomes First European Approved Biologic Il 12 Il 23 Inhibitor For Ulcerative Colitis Pharmafile
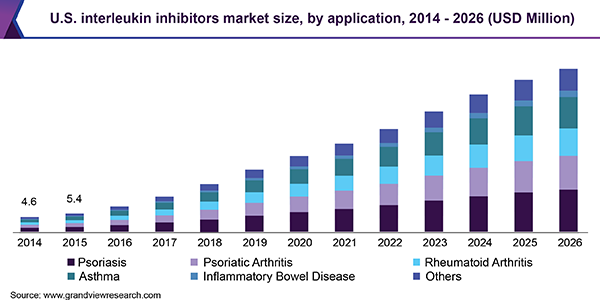



Il Inhibitors Market Size Share Industry Trends Report 19 26



Pathogenic Role Of Cytokines And Effect Of Their Inhibition In Psoriasis Intechopen



Drug Survival Of Il 12 23 Il 17 And Il 23 Inhibitors For Psoriasis Treatment A Retrospective Multi Country Multicentric Cohort Study Springerlink




Management Of Plaque Psoriasis A Review And Comparison Of Il 23 Inhibitors European Medical Journal




Janssen S Tremfya Shows Promise In Psa Patients With Sacroiliitis Drug Discovery And Development




Target Processes Or Pathways Of New Drugs With Evidence For Clinical Download Scientific Diagram




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect




Experimental And Investigational Pharmacotherapy For Psoriatic Arthrit Jep




Ustekinumab And Anti Interleukin 23 Agents In Crohn S Disease Gastroenterology Clinics




Therapeutics Targeting The Il 23 And Il 17 Pathway In Psoriasis The Lancet




Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology




Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology



Full Article Monoclonal Antibodies Inhibiting Il 12 23 And 17 For The Treatment Of Psoriasis




From Evolution To Revolution Il 23 In The Treatment Of Psoriasis Patients European Medical Journal




New Biologics And Small Molecules Under Development For The Treatment Download Scientific Diagram




Fda Approves Guselkumab For Psoriatic Arthritis




Targeting P19 As A Treatment Option For Psoriasis An Evidence Based R Tcrm




Janssen Files Stelara Follow Up Guselkumab In Europe Pmlive



1




Boehringer Says Anti Il 23 Drug Beats Stelara In Psoriasis Trial Pmlive



0 件のコメント:
コメントを投稿